The following 30-second video demonstrates how our technology works:
Intellectual Property
TheraBiome owns five issued US patents and 19 international patents on its proprietary platform technology. Patents protect drug and biologic delivery, including vaccines.
Differentiated Drug Delivery Target Product Profile (TPP)
- TheraBiome's GEMICEL® platform technology is Phase II-ready based on its strong science and human proof-of-concept clinical studies compliant with FDA and international regulatory standards.
- This technology is suitable for delivery of small molecules and biologics including vaccines.
- Longer acting and locally delivered.
- TheraBiome is ready to support CMC strategy and deliverables.
- Disease and therapeutic area agnostic despite special focus on GI-related indications.
- Easy to swallow - patient compliant products.
Phase 2-Ready Precision GI Targeting
GEMICEL® Oral Delivery System
The gastrointestinal (GI) tract’s heterogeneous pH and chemical milieu poses challenges to ensuring targeted delivery of therapeutics into physiologically relevant gut segments using traditional technology. Some therapeutics may be ineffective when taken orally; they may be too sensitive, incompatible, subject to high first-pass metabolism or require higher doses. As a result, the formulation may fail to demonstrate efficacy and/or safety. GEMICEL enables precise and site-specific targeted delivery with controlled release using a proprietary oral formulation, maximizing chances of success in meeting target product profile in clinical trials. All formulation components comply with global safety standards, and the technology has been successfully scaled up. TheraBiome is seeking partners who may benefit from precision GI targeting.
Phase 2 Ready
Scintigraphy studies with multiple cohorts were conducted in 27 healthy completed for technology validation. Product scale-up was completed. Phase IB studies were initiated in 24 subjects before Assembly discontinued the ulcerative colitis study following AbbVie’s acquisition of Allergan
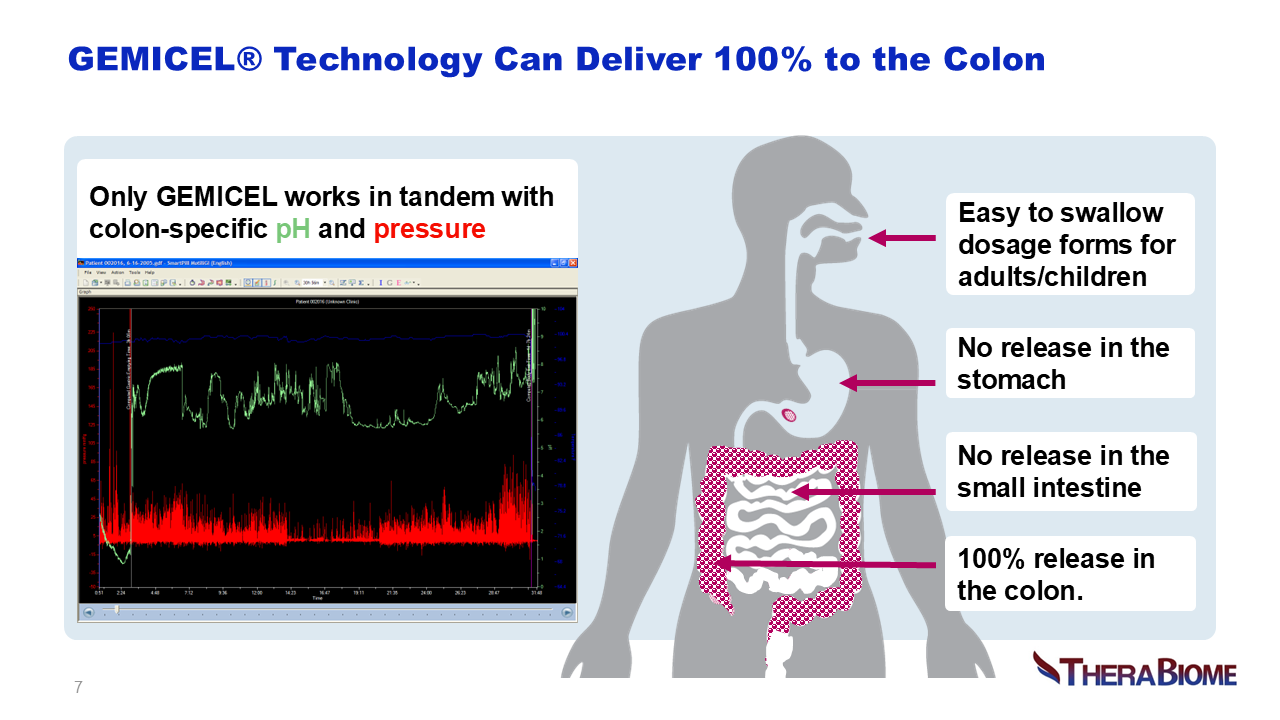
Unmet Medical Need: Oral Targeted Enteral Delivery
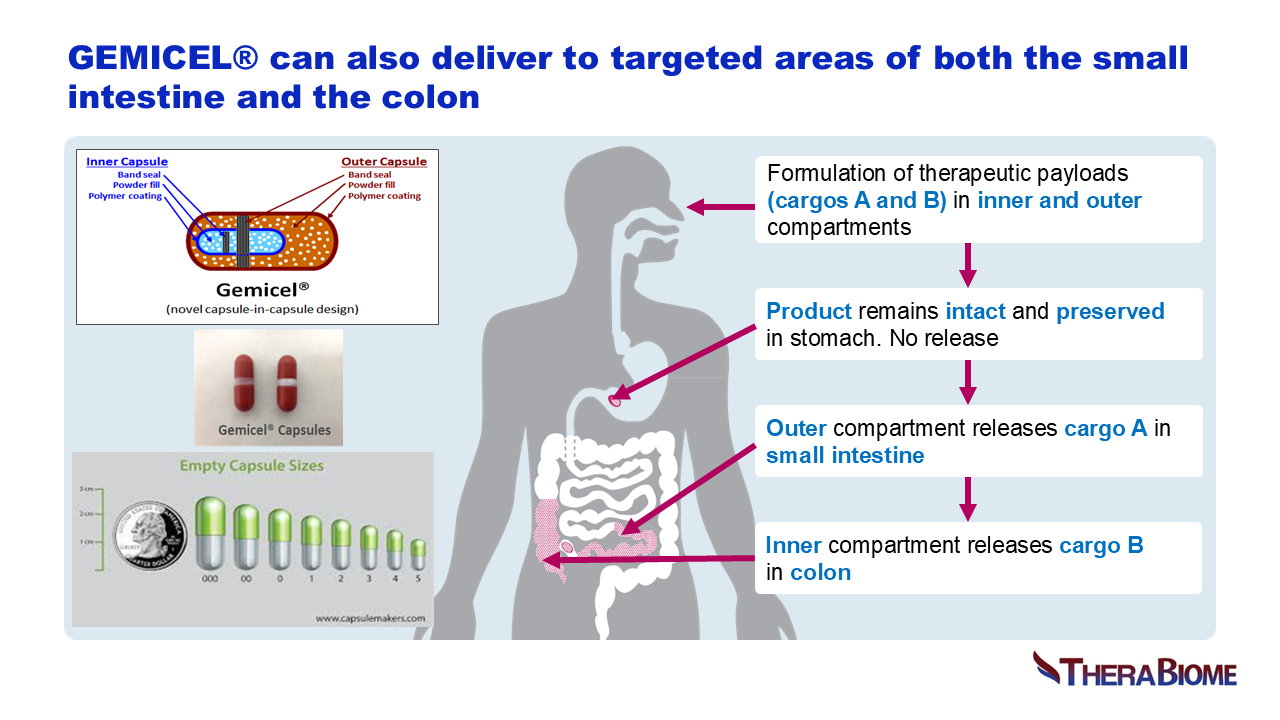
Targeted oral delivery technologies include conventional pH-sensitive enteric coatings, multi-matrix, osmotic-controlled and timed-release methods. Limitations include variable or off-target delivery, reduced potency, leakage from one site to another, lack of patent protection and manufacturing complexity, as well as high COGS. GEMICEL overcomes these challenges cost-effectively based on its dual-targeted drug delivery platform. Human clinical scintigraphy studies confirmed that GEMICEL is colon targeted, highly tunable and reliably delivers high therapeutic payloads to the colon alone, or to a combination of the small intestine and the colon. This patented delivery technology is based on dual polymer layers programmed to dissolve at specific pH and pressure levels in capsules or tablets. GEMICEL in various oral forms delivers small molecules and oral biologics including proteins, peptides, and vaccines (providing mucosal immunity) to specific target locations in the GI tract. This technology is specifically designed to target the microbiome with live biotherapeutic products that would otherwise struggle to achieve their intended effects. As a gut-restricted innovation, it effectively addresses unmet medical needs.
We need your consent to load the translations
We use a third-party service to translate the website content that may collect data about your activity. Please review the details in the privacy policy and accept the service to view the translations.

